Physical Properties of Group 1 Elements
Physical Properties of Group 1 Elements: Overview
This topic covers concepts, such as, Physical Properties of Alkali Metals, Density of Alkali Metals, Hydration Energy of Alkali Metals & Sublimation Enthalpy of Alkali Metals etc.
Important Questions on Physical Properties of Group 1 Elements
Which of the following trends decreases down the group in alkali metals?
Which of the following alkali metal has highest melting point?
Assertion : Alkali metals are soft and have low melting and boiling points.
Reason : This is because interatomic bonds are weak.
The salt of an alkali metal gives yellow colour in the flame test. Also, its aqueous solution gives an insoluble white precipitate with barium chloride in acid medium. The salt is
Which of the following property is not a characteristic of alkali metals?
Which of the following elements has the maximum density?
Which of the following property is not a characteristic of alkali metals?
Which of the following elements has the maximum density?
The most stable carbonate is
Which one of the following properties increases on moving down the group from Li to Cs ?
In aqueous solutions, which of the following properties indicates the strong reducing character of lithium?
Alkali metal salt gives a pale violet colour in flame test is:
Low solubility of in water is due to:
Which of the following increases in magnitude as the atomic number of alkali metals increases?
The hottest region of the Bunsen flame shown in the figure below is:
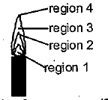
Compounds of alkaline earth metals are less soluble in water than the corresponding alkali metal salts due to:
Which of the following will appears silvery-white?
The metallic lustre exhibited by sodium is explained by
Which of the following block metals do not impar any colour to the flame
Weak reductant in alkali metal is:-
